Protecting Future Generations ; Lessons From The Past
Introduction
Sodium Valproate is an anti-epileptic and mood-stabilising medication that has been widely prescribed in the UK since the 1970s. While effective in managing seizures and bipolar disorder, the drug poses a severe teratogenic risk when taken during pregnancy, with up to 4 in 10 babies exposed in the womb developing physical and developmental disabilities.
For decades, the risks were not adequately communicated to women of childbearing age, leading to a public health catastrophe now known as the Sodium Valproate Scandal. As a direct result of tireless campaigning, most notably by INFACT and its co-founders – the UK has made important progress in establishing national prescribing safeguards.
The Turning Point: Panorama and the INFACT Campaign
On 30th June 2013, the BBC’s Panorama programme “The Truth about Pills in Pregnancy” exposed systemic failures in the regulation and prescribing of Sodium Valproate. This investigation, built upon years of evidence gathered by INFACT and affected families, marked a turning point in public awareness and political accountability.

August 2013 – Dr P Turnpenny, Emma Murphy, Janet Williams, Dr J Raine, S Morgan, S Mee
Following the programme and under mounting pressure, the Medicines and Healthcare products Regulatory Agency (MHRA), quickly invited INFACT to a meeting at there head office to hear our concerns about the need for stricter regulation with Valproate. This meeting led directly to the MHRA setting up the Valproate Stakeholder Network where INFACT and other patient groups were able to develop and implement stricter prescribing protocols.
Current Prescribing Guidelines
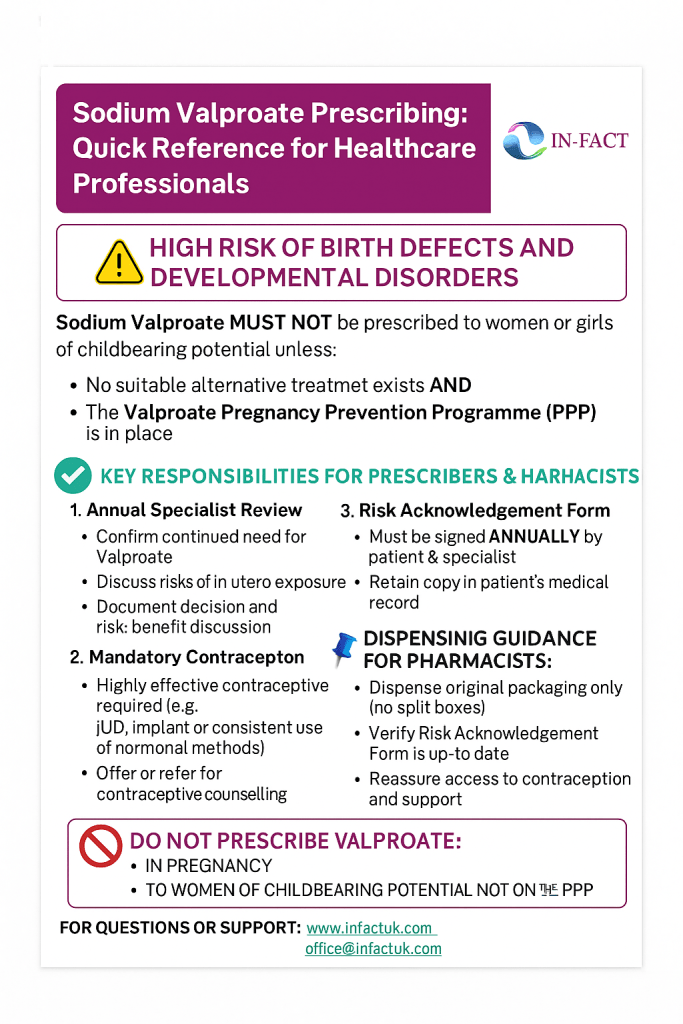
The updated guidelines were introduced to protect women of childbearing potential and prevent further harm. The key elements include:
2. Pregnancy Prevention Programme (PPP)
All women of childbearing age who are prescribed Sodium Valproate must be enrolled in the Pregnancy Prevention Programme unless there is no alternative treatment, and they are fully informed of the risks.
Key PPP components:

- Regular specialist reviews (at least annually)
- Contraceptive counselling and provision
- Signing of a Risk Acknowledgement Form
⚠️ What Women Taking Sodium Valproate Need to Know
Your safety, your choices – informed, supported, and protected
| What You Need | Why It Matters | What You Should Expect |
|---|---|---|
| Annual review with a specialist | Valproate can harm unborn babies. Your treatment must be reviewed regularly to check if it’s still the best option. | You should see a neurologist, psychiatrist, or other relevant specialist at least once a year. They will talk to you about your treatment and the risks. |
| Risk Awareness Discussion | You deserve clear information about how Valproate can affect pregnancy. | Your healthcare provider should talk to you about the risks before you’re prescribed Valproate and every year after that. |
| Signed Risk Acknowledgement Form | This ensures you’ve been given all the information and supports shared decision-making. | You and your specialist should sign this form each year. It confirms you understand the risks and your options. |
| Effective contraception | Valproate can cause birth defects and developmental conditions. Preventing pregnancy while on Valproate is essential. | You should be offered highly effective contraception (like an IUD, implant, or pill). Support for contraception should be easily accessible. |
| Valproate Patient Guide | You have the right to written information in plain language. | Your GP, pharmacist, or specialist should give you a printed guide every year. You can ask for another copy at any time. |
| Clear packaging with warnings | Every woman and girl taking Valproate should be reminded of the risks with every prescription. | Your Valproate should come in its original box, with a visible warning label. Don’t accept plain packaging. |
All women of childbearing age who are prescribed Sodium Valproate must be enrolled in the Pregnancy Prevention Programme unless no suitable alternative treatment is available and the woman has been fully informed of the risks.
Key PPP components include:
- Specialist Review: Women must be reviewed at least annually by a specialist, with the need for Valproate reassessed.
- Contraception Requirement:
Women must be on highly effective contraception throughout treatment with Valproate. This is a mandatory condition of the PPP.
Accepted methods include:- Long-acting reversible contraception (LARC), such as the intrauterine device (IUD) or implant
- Or combined hormonal methods, when used reliably
The aim is to ensure that pregnancy does not occur while taking Valproate, given the high risk of serious harm to the unborn child.
- Contraceptive Counselling: Women must receive counselling on suitable contraceptive methods, either via a specialist or sexual health professional.
- Risk Acknowledgement Form:
Both the patient and prescriber must sign this form every year to confirm that the risks of taking Valproate during pregnancy have been discussed and understood, and that contraception is being used appropriately.
3. Prescribing Restrictions
Sodium Valproate must not be prescribed:
- To women or girls of childbearing potential unless other treatments are ineffective or not tolerated
- To pregnant women for any reason unless there is no suitable alternative
Prescribers and pharmacists are legally required to follow strict dispensing protocols, including adding visual warnings on packaging and ensuring no woman is prescribed Valproate without a documented risk discussion.
Valproate Guide for Women : https://www.medicines.org.uk/emc/rmm/104335/Document
Valproate Guide for Men : https://www.medicines.org.uk/emc/rmm/104334/Document
The Role of INFACT in Driving Change
INFACT’s advocacy was instrumental in bringing about these reforms. From gathering testimonies to engaging the media, Parliament, and healthcare regulators, our campaign ensured that the voices of affected families were finally heard.
These guidelines exist today because of decades of persistence from grassroots campaigners and mothers who refused to remain silent while preventable harm continued.
Ongoing Concerns
Despite progress, there remain serious challenges:
- Inconsistent implementation of the PPP across trusts and GP practices
- Lack of routine audits to ensure compliance
- Inadequate follow-up for women already affected or misinformed
INFACT continues to call for:
- Mandatory training for all healthcare professionals involved in prescribing
- Independent oversight of PPP implementation
- Lifelong support for affected families
Resources & Further Information
Conclusion
The prescribing guidelines for Sodium Valproate mark a critical step forward in protecting women and future generations. However, their effectiveness relies entirely on proper implementation, monitoring, and accountability. INFACT remains committed to ensuring that no more families are left in the dark and that justice, awareness, and support continue to grow.
Copyright Emma Murphy, Janet Williams – Independent Fetal Anti convulsant Trust

